Scandinavian bronzecasting in the Viking Age and the Early Middle AgesAnders Söderberg
There's also a more elusive fascination behind my interest in the subject. It started with a fascination for the vitrified goods in crucibles and melted clay, left behind by the process. The glassy surfaces, coloured in black over green to red, tell stories of brutal heat and blowing bellows a long time ago - but with a very tangible presence. Maybe this presence is created by the fact that hard-burnt and vitrified clay doesn't change while lying in soil for centuries, like other archaeological materials do. The sintered ware is stable and hardy and after being digged-up and washed, a viking age crucible looks the same today; just the way it looked when cooling-off at the workshop's floor a thousand years ago. Even Bronze-Age crucibles haven't changed very much, despite the passing of three thousand years. This gives a perspective on time. It's a strong reminder, of thousand or three thousand years being no more than a snap with your fingers in the universe. The presence of ancient times becomes even more tangible, when I see my own crucibles melt down into glassy melts very similar to the ones I can watch in the depositories of the National Museum of Antiquities. Making the same mistakes as the early medieval craftsmen, makes history less extraordinary and pull it a bit closer to you. Bronze casting is an elegant play with a couple of cubic-decimetres of borrowed hell. It's quite handy as it's limited to a small pit, but deceptive as you easily could be seduced to think you're its master. Who's terms you're working under is obvious each time you accidentally put your thumb too close to the hearth, or when you in distraction almost grips the crucible with your fingers. These things bite, and they bite bad.
Early medieval casting had deep traditions since Bronze Age. The Viking Age process probably looked quite the same as it already had done for a couple of thousand years. If Bronze Age was the golden age of bronze casting, the craft didn't die with the coming of iron. Metal casting still played an important social part by the production of jewellery and prestige objects, a production of social codes telling about identity and belonging; of sworn fidelity and of alliances. All confirmed by a system of gift giving, almost strong enough to give each object a life of its own; at least this may have been the way contemporary man regarded it. We've been able to see a part of this when excavating Iron Age and early medieval graves. We ourselves have benefited from parts of these forgotten code systems, as the burial gifts still tell stories about the persons buried. Since a couple of decades, we've also found many early medieval workshops in Sweden; in the Migration Age settlement at Helgö in Lake Mälaren; in the medieval towns of Lund and Sigtuna; in Viking Age town Birka and at several places in Denmark. Here we've been able to create an understanding in the craftsmen’s conditions and - more tangibly - an understanding in the technical aspects of the different crafts themselves.
I've used the last three years (1998) to penetrate some of these crafts. I've particularly worked with bronze- and silver-casting using help provided by excavation publications and earlier experimental projects. Danish and English archaeologists holds a forward position in this matter, and I'm grateful for the good documentation that is available (Bareham 1994) (Hedegaard 1992) (Johansson 1986) (Kruse, Smith & Starling 1988) (Lønborg 1986 & 1992) (Tylecote 1973).
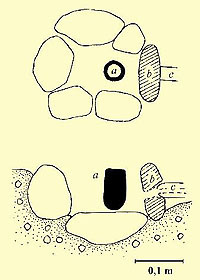
The hearthThe heart of the workshop is the charcoal hearth. The hearths I've been working with have had their patterns in the small bronze-casting hearths from Helgö, excavated in the fifties and in the sixties (Lamm 1973) (Lamm & Clarke 1984). A hearth doesn't have to be large, especially since each casting seldom demands more than 40-50 cm3 of bronze, often less than this. I've usually been working with hearths of 0.001-0.002 m3 in size. Otherwise, a common Viking Age type of hearth for melting metal seem to be a simple clay-walled pit in the ground, slightly larger than the hearths I've been using (Roesdahl 1977) (Lønborg 1986)
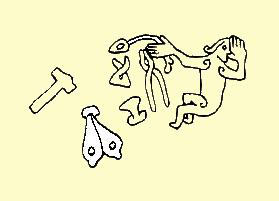
A pair of bellows is connected to the hearth. Like the hearth itself, these don't have to be large. The bellows I've built are made from the pattern of a picture in a runic carving; the Sigurd (Sigfried) Runic Carving, Sweden. Since the carver shows a relatively good fidelity to proportions when picturing Sigurd's tools, I've used the carving almost as a direct pattern for my pair of bellows. They measure approx. 0,4 meters in lenght and approx. 0,2 meters in width (each bellow). This size is quite enough to create an efficient heat-centre of approx. 0.001 m3 and the size of the hearth is determined by this effect. The bellows must be used in pairs, to prevent a situation of gases going backwards into the bellows chamber, causing a risk of explosion. This is a small but efficient system. With a steady, and actually quite laid-back, pumping it's easy to reach the necessary 1100-1200 degrees C and a crucible filled with bronze can be melted in 15 minutes.
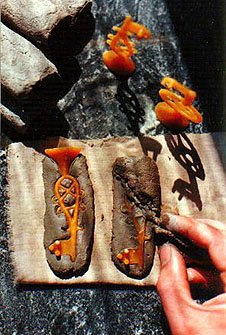
The moulds Since the Bronze Age, a common type is a mould of clay tempered with fine sand and some organic material; cattle- or horse spilling is excellent as the fibres in such material are cut into ideal lengths of 1-5 mm. There have been a discussion in Scandinavia, since the early fourties, about how these moulds were made and which casting-technique they derive from (Oldeberg 1942, 1948 & 1966) (Zachrisson 1960) (Lamm 1980) (Thunmark-Nylén 1983) (Brinch Madsen 1984) (Jansson 1985). The main standpoints in the discussion have been that that either the moulds were formed by pressing an original metal object into the moulding material and thereafter the two mould halves placed together or that they were derived from a lost-wax method. A common, diplomatic, middle-of-the-road conclusion among archaeologists has been that both methods were used. Danish experimental archaeology have seized upon lost-wax casting - á cire perdue (Lønborg 1986 & 1992). In lost-wax casting you bake the wax original into the mould material, and melt it out after the mould have been dried. Wax models can easily be reproduced by pressing the original into clay, after which the imprint is filled with wax (Hedegaard 1992). This is a rewarding method, creating models with relatively good fidelity to the original object. Even objects with a certain overlap in the ornaments can be reproduced in this way, as the plasticity in the wet clay allow you to bend it out a little when removing the original object. The advantages of the lost-wax technique lies in allowing castings with high fidelity to the original and giving a remarkable fine detailing. Another advantage is that necessary retouch in the ornaments can be made in the soft wax, instead of making this in the final metal object. A third advantage is the possibility of a fast mass production provided by this method (Hedegaard 1992). By the help of one metal original, you can quickly make as many wax models as you want, and easily make at least twenty moulds within a few hours - depending on the need for retouch claimed by the object you're going to cast. Objects with intricate ornaments need more retouching than objects of simpler shape.
The cire perdue-method was in the Early Middle Ages already a couple of thousand years old and it's still alive today, in ethnic foundry and in jewellery and art foundry as well in precision steel foundry. In the later case special waxes and hi-tech ceramics are used, but the principle is the same as in the Middle East maybe tree thousand years BC - a high technological industrial process leaning upon four or five thousand years of low technological tradition.
To me, it seems quite natural to associate plastic and water-soluble mould materials to lost-wax casting. My hypothesis regarding the ancient clay moulds is that they generally derive from a process like this. I don't think every archaeologist will agree on this, but I am presently working on trying to confirm it. Maybe I will have to surrender in the end, but in that case my hypothesis at least will have been a good research platform.
In archaeology we often carelessly speak of all copper alloys as "bronze", but it's merely classic bronze, with 90 % copper (Cu) and 10 % tin (Sn) and close related alloys, that can claim this name.
When talking about Viking "bronzes", we're actually often talking about alloys closer related to present-time brasses. Brasses are alloys of 30-40 % of zinc (Zn) added to the copper. During the first centuries BC, the Romans worked out methods of mass producing brass. At this time it wasn't possible to smelt pure zinc ore, but it was possible to mix metallic copper with zinc ore and heating it until the zinc vaporised and got absorbed into the copper. The method is known as a cementation process. (Craddock 1990)
This alloy quickly grew popular and the prize rose several times higher than the copper price in Rome. The Romans founded brass foundries in present Belgium and Germany in the region around Aachen. From the continent metal was imported to Scandinavia, as ready-made objects, as scrap-metal and probably as raw material in rods. The production seems to have survived the falling of the Roman Empire in the 5th century, and it rose again in Carolingian management in the 9th century AD. (Craddock 1990)
Copper-zinc alloys have the advantages before the rather slowly flowing tin-bronzes in being low viscous and in solidifying slower. With these alloys you easily get a good filling in the mould, out to the finest details. The low viscosity also allows you to pour a very fine metal squirt into the moulds sprue while letting gases stream out beside. Viking age moulds are rarely known to be supplied with air channels.
Pure copper-zinc alloys were rarely used in Migration- Viking- and Early Middle Age Scandinavia. There often was an adding of tin or lead (Pb) or both. A correct English terminology for these alloys, would be gunmetal (brass + Sn) leaded brass (brass + Pb), and leaded gunmetal (gunmetal + Pb). Modern analyses of Scandinavian Early Middle Age bronzes show, in their most homogenous results, alloys of Cu + c. 10-25 % Zn and c. 5-15 % of Sn/Pb. We can see, in analyses, a slight pattern of moving towards leaded gunmetal’s, with an increasing addition of Pb during Viking Age (Arrhenius 1989) (Forshell 1992)
It could be that these additions have been added at a local craftsman-level. After several re-melting’s of the alloy the zinc would have boiled away and the metal had became more copper and less brass, and therefore more viscous and useless for casting. This is the point where I suggest that the lead and tin, which might not have been part of the original raw material, could have been added. The adding could have been made in order to make the alloy useful for casting, despite a high loss of zinc. This could of course have taken place as well at a continental craftsman-level, as in Scandinavia later in the chain of distribution. Contents of Pb and Sn have been detected in Roman and in Provincial Roman iron age objects as well (Dungworth 1997).
The idea of adding Sn/Pb on local craftsman-level is so far much of a speculation. A content of Pb in the alloys can also be a result of impurities in zinc ores; the use of ores containing lead.
The crucibles
Like the moulds, the crucibles were made from sand-tempered clay. Domestic Scandinavian clays of earthenware qualities are not very resistant to the heat of the melting hearths, especially since the charcoal contains fluxing compounds – like potassium and natrium compounds - lowering the clay's melting point dramatically. The craftsmen solved this problem by tempering the clay with large quantities of sand or ground quartz (Lamm 1973 & 1980). Quartz has a high melting point, at about 1700 degrees C. Despite this, even the quartz grains' surfaces slightly start to melt in this tough environment.
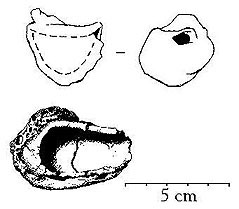
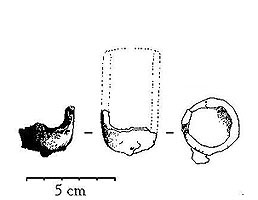
Migration-age lidded crucibles from Helgö. Below broken, lid missing. Viking-age thimbleshaped crucible, fragment from Sigtuna. After the metal has been melted in hearth, the actual pouring can take place. Prior to this, the mould is burnt in a separate hearth. The best casting results are given in moulds completely burnt-through and oxidised - the ceramic material must be red-burnt all way through. If the mould's goods still is black or grey, it contains remains of organic temper, wax and unburnt lime from the lime-carrying Scandinavian glacial and post glacial clays. Lime developes carbon dioxide when burning. Under these conditions the mould emits quantities of gases enough to obstruct the casting - sometimes the emission of gases is large enough to even make it impossible even to pour any metal into the mould. This have caused a lot of puzzle while investigating the casting technique - especially since ancient moulds often are quite poorly burned; sometimes the moulds are red-burnt on the surfaces but deep black in the goods. How did they succeed to even pour a small droplet of metal into these moulds? Especially since this often is the case in moulds for the Viking Age oval brooches; objects of a millimetre-thin goods which demands great skill to cast even under the best conditions?
Along with these moulds there also are lots of well-burnt moulds that rarely would have caused any problems, but the amount of black-reduced moulds is large enough to create confusion. Did the Viking founders really mass produce failed castings? Nevertheless, it seems like the zinc-copper alloys used at the time are a little more tolerant against mould gases than the tin-bronzes, and when casting in similar alloys you are able to keep a lower mould temperature. A lower temperature in the moulds creates less gas. I'm trying to investigate these matters more closely. Perhaps analyses of black moulds would show the access to, and use of, less lime-containing clays. Nevertheless - lime or not - the high charcoal content in a black-reduced mould, must affect the casting anyway.
The hearth in which the moulds are burnt doesn't have to be blast with bellows. With good ventilation to the hearth it's easily possible to reach the 700-800 degrees C needed, burning them and keeping them hot until casting.
The mould clay seems to be the ideal material for this matter. Clay is a slow heat conductor. It demands a long time for heating and a long time for cooling off. A mould with 5 mm cavity walls needs more than 15 minutes to be heated all way through from room temperature up to 700 degrees C. These conditions give the possibility of taking the mould out of hearth before casting. It's possible to hold it in tongs in your left hand, which strengthen the mobility of your hands when casting. An interval of twenty seconds is given before a 700 degrees C mould slowly starts to cool off after being taken out of hearth. Twenty seconds is a good deal of time, in a context where most things normally must be done with a good speed.
The slow heat conduction of the clay also creates a situation, where the heat of the liquid metal to a very small extent is transported through the mould ware. It doesn't primarily seem to be cooled down so much by the mould itself, especially since the mould is pre-heated. This is a good help when casting objects with fine ornaments and thin details. It seems like most of the heat is conducted up through the metal itself, and out through the metal plug created in the sprue, rather than out through the mould material.
The early medieval founder's achievements were no wonders, though contemporary opinions would certainly have thought so. Their skills were dependent on knowledge based on long traditions and good methods combined with the knowledge to solve problems in easiest possible ways; but with keeping of optimal results. My experience - so far - is that clay moulds tempered with sand and cattle dung in combination with good alloy’s, could help you create quite a lot. Sometimes even amazing things.
References
Arrhenius, B. 1989. Kan metallanalyser ge en anvisning om när metallurgi blir inhemsk? In: Icke-järnmetaller - malmfyndigheter och metallurgi. Jernkontorets bergshistoriska utskott , H 45. Stockholm.
Bareham, T. 1994. Bronze casting experiments. In: Historical Metallurgy - The Journal of the Historical Metallurgy Society, Vol 28 No 2, 1994. London.
Brinch Madsen, H. 1984. Metal Casting. In: Ribe Excavations 1970-76. Vol 2. Bencard, M. (Ed). Esbjerg.
Craddock, P. T. (Ed). 1990. 2000 Years of Zinc and Brass. Occasional paper No 50. British Museum. London.
Drescher, H. 1983. Metallhandwerk des 8.-11. Jh. in Haithabu auf grund der Werkstattabfälle. In: Das Handwerk in vor- und frühgeschichtlicher Zeit. Teil II, Achäologische und philologische Beiträge. H. Jahnkuhn (Ed.). Göttingen.
Dungworth, D. 1997. Iron Age and Roman copper alloys from northern Britain. In: Internet Archaeology 2 (http://intarch.ac.uk/journal/issue2/dungworth_index.html). A. Vince (Ed.). Dept of Archaeology. University of York.
Forshell, H. 1992. The inception of copper mining in Falun. Relation between element composition in copper artefacts, mining and manufacturing technology and historic development with particular emphasis on copper from the Falu mine. Theses and papers in archaeology B:2. Stockholms Universitet. Stockholm.
Floderus, E. 1928. Några brons- och silversmedsfynd från det äldsta Sigtuna. In: Fornvännen 23 (1928). Curman, S (Ed.). KVHAA. Stockholm.
Hawthorne, J. G. Smith, C. S. 1979. Theophilus; On Divers Arts. The Foremost Medieval Treatise on Painting, Glassmaking and Metalwork. New York.
Hedegaard, K. R. 1992. Bronzestøberhåndværket i yngre germanertid og tidlig vikingetid i Skandinavien - teknologi og organisation. In: Lag 1992. Højbjerg.
Jansson, I. 1985. Ovala spännbucklor. En studie av vikingatida standardsmycken med utgångspunkt från Björköfynden (Oval brooches. A study of Viking Period standard jewellery based on the finds from Björkö (Birka) Sweden). Aun 7. Uppsala Universitet. Uppsala.
Johansson, T. (Ed.). 1986. Brons och koppar. Forntida teknik 12. Sveg.
Kruse, S. E. Smith, R. D. Starling, K. 1988. Experimental casting of silver ingots. In: Historical Metallurgy - Journal of the Historical Metallurgy Society, Vol 22 No 2, 1988. London.
Lamm, K. 1973. The Manufacture of Jewellery during the Migration Period at Helgö in Sweden. In: Bulletin of the Historical Metallurgy Group. Vol 7, no 2. London.
Lamm, K. 1980. Early Medieval Metalworking on Helgö in Central Sweden. In: Aspects of Early Metallurgy. Oddy, W. A. (Ed.). British Museum Occasional Paper No 17. London.
Lamm, K. Clarke, H. (Ed.).1984. Excavations at Helgö IX. Finds, Features and Functions. KVHAA. Stockholm.
Lindblad, K. G. 1997. Metallgjutning med forntida metoder. In: Nytt om smide och smeder April 1997, Nyhetsbrev för Sveriges Konstsmidesförening.
Lønborg, B. 1986. Bronzestøbning i dansk jernalder. In: Kuml 1986. Årbog for Jysk Arkæologisk Selskab. Kjærum, P. (Ed.). Aarhus.
Lønborg, B. 1992. Fremstillingen af vikingetidens skålformede fibler. In: Kuml 1992. Årbog for Jysk Arkæologisk Selskab. Kjærum, P. (Ed.). Aarhus.
Nordin, A-C. 1993. Metallgjutning i Sigtuna. C-uppsats. Inst. för Arkeologi, Uppsala Universitet. Uppsala.
Oldeberg, A. 1942-1943. Metallteknik under förhistorisk tid I-II. Lund.
Oldeberg, A. 1948. Sandgjutning eller á cire perdue? In: Fornvännen 1948. KVHAA. Stockholm.
Oldeberg, A. 1966. Metallteknik under vikingatid och medeltid. Stockholm.
Roesdahl, E. 1977. Fyrkat - En Jysk Vikingeborg, II Oldsagerne og Gravpladsen. København.
Tesch, S. (ed.) 1990. Makt och människor i kungens Sigtuna - Sigtunautgrävningen 1990. Sigtuna Muséer. Märsta.
Thunmark-Nylén, L. 1983. Vikingatida dosspännen - teknisk stratigrafi och verkstadsgruppering. Aun 4. Uppsala Universitet. Uppsala.
Tylecote, R. F. 1973. Casting Copper and Bronze into Stone Moulds. In: Bulletin of the Historical Metallurgy Group, Vol 7 No 1, 1973. London.
Zachrisson, I. 1960. De ovala spännbucklornas tillverkningssätt. In: Tor VI. Uppsala.
|
©Text & images: Anders Söderberg [infoATvikingbronze.com] April 30 1999 |
.